
Personalized K-TrackTM Assay to Detect Actionable Mutations and Minimal Residual Disease in Solid Tumors
Authors
BACKGROUND
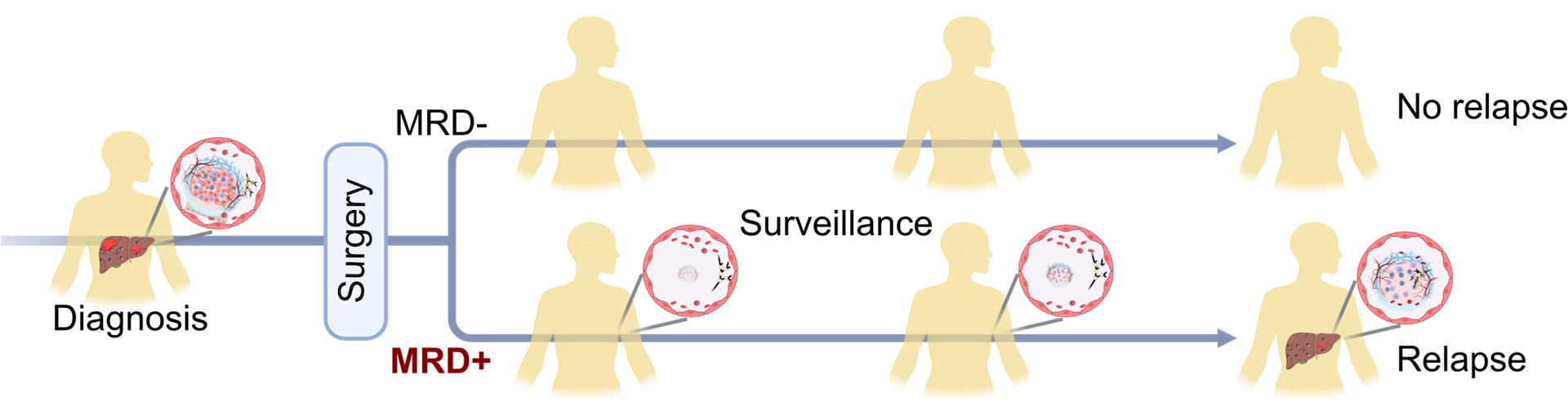
- Minimal residual disease (MRD) refers to a small number of cancer cells that remain in the body after curative-intent treatment, causing relapse and metastasis. Current tumor biomarkers and imaging methods have limited sensitivity and specificity to monitor MRD for solid tumors.
- Circulating tumor DNA (ctDNA) in liquid biopsy has emerged as a promising biomarker to monitor MRD.
- Current MRD assays remain mostly inaccessible and unaffordable for patients in developing countries.
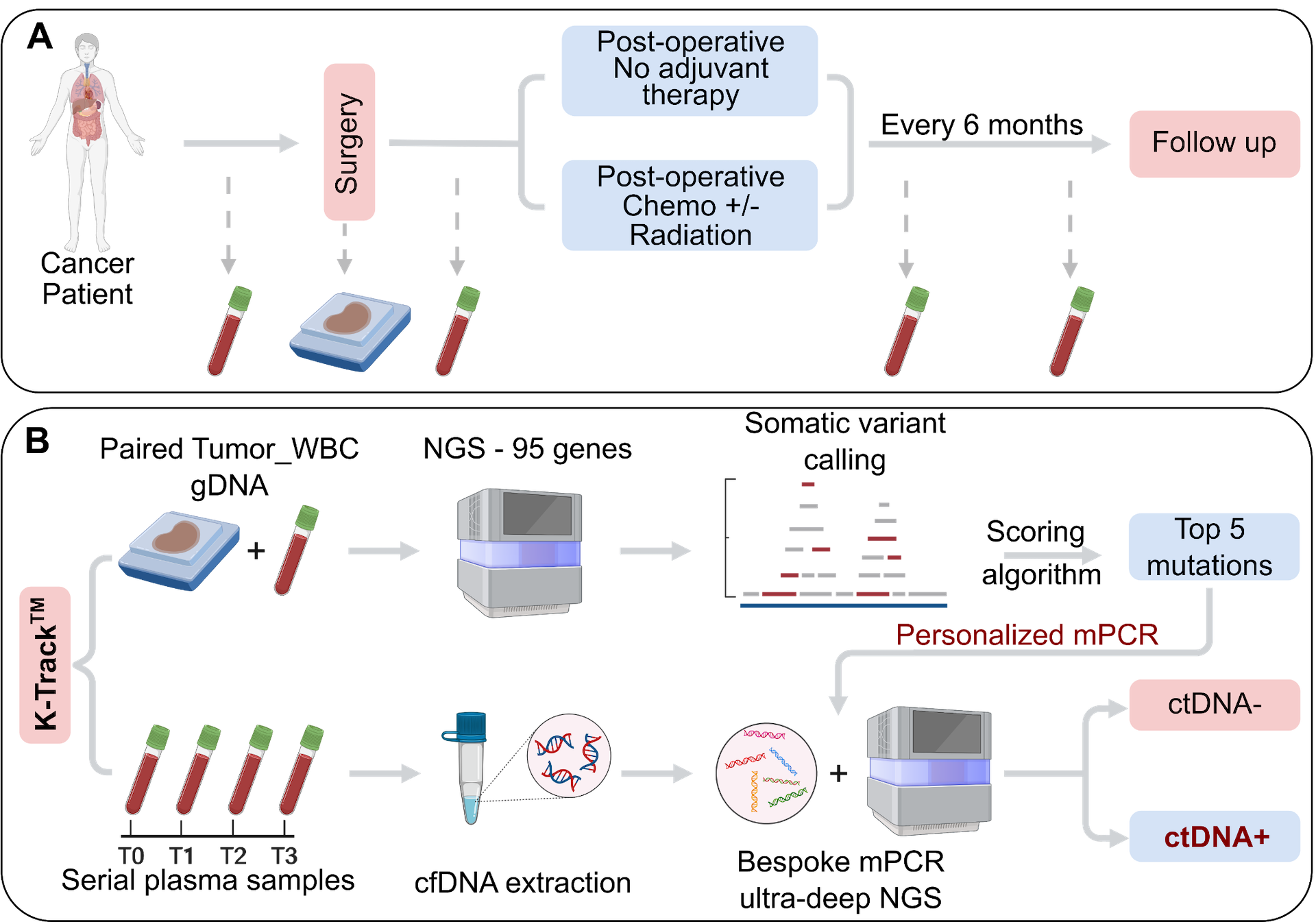
METHOD
- Tumor-informed personalized ctDNA tracking assay
- Streamlined and cost-effective
PRE-OPERATIVE ctDNA DETECTION
Table 1. Detection of ctDNA in plasma samples before radical surgery
| Cancer type | Cohort (N) | Detection rate | |||
|---|---|---|---|---|---|
| Hepatocellular carcinoma (HCC) | 60 | 96.6% | |||
| Colorectal Cancer (CRC) | 115 | 85.2% | |||
| Breast Cancer (BC) | 115 | ||||
| HR(+), HER2(+/-), high risk* | 70 | 40.0% | |||
| HR(-), HER2(+) | 30 | 83.3% | |||
| HR(-), HER2(-) | 15 | 80.0% | |||
| Non-small cell lung cancer (NSCLC) | 50 | 62.0% | |||
| Gastric Cancer (GC) | 94 | 45.7% | |||
| Ovarian Cancer (OC) | 17 | 64.7% | |||
(*) High risk: ≥15% predicted risk of death within 10 years using ePREDICT V2.1, or tumor size T>5 cm, or N≥4 nodes, or N=1-3 nodes and at least one of the following: T>3 cm, grade 3, high genomic risk defined as Oncotype Dx Recurrence Score >26
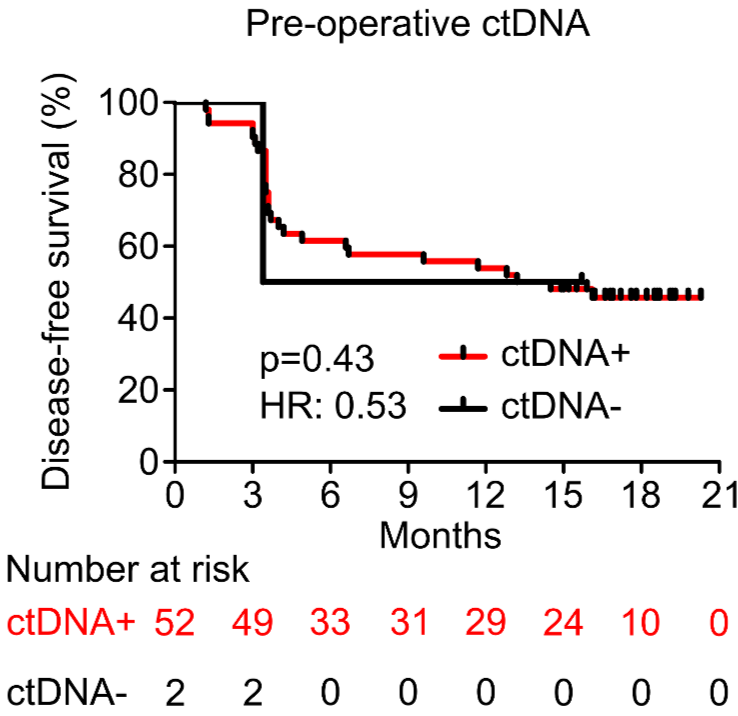
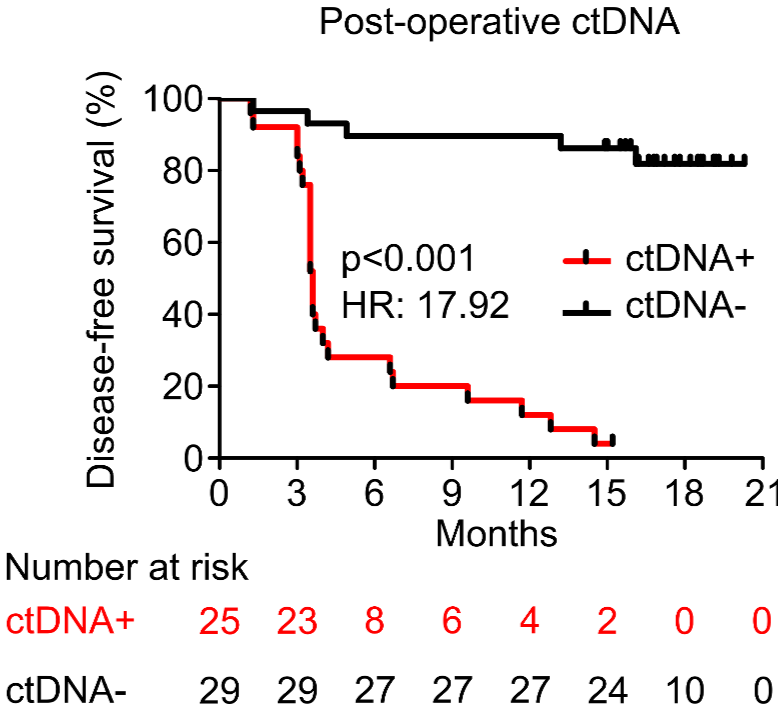
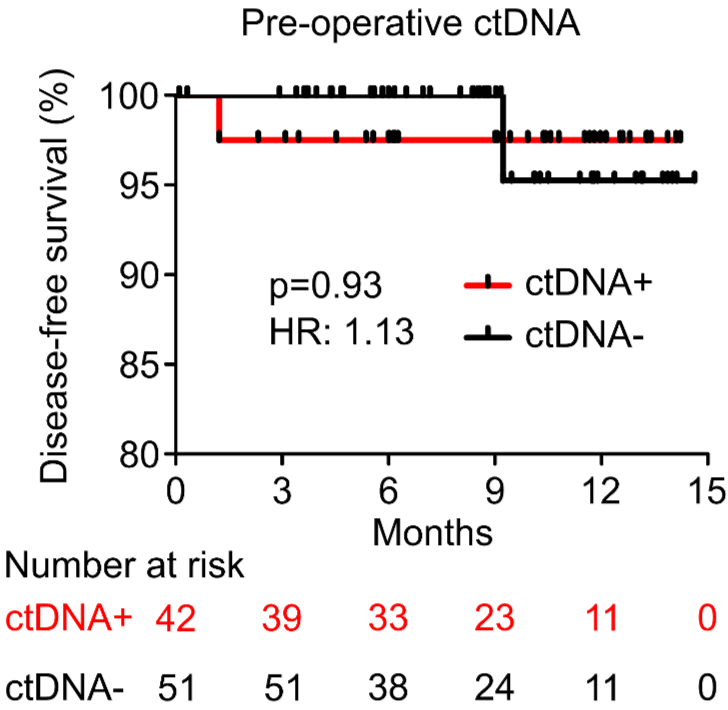
PROGNOSTIC VALUE OF POST-OPERATIVE ctDNA
A - Hepatocellular carcinoma (HCC)
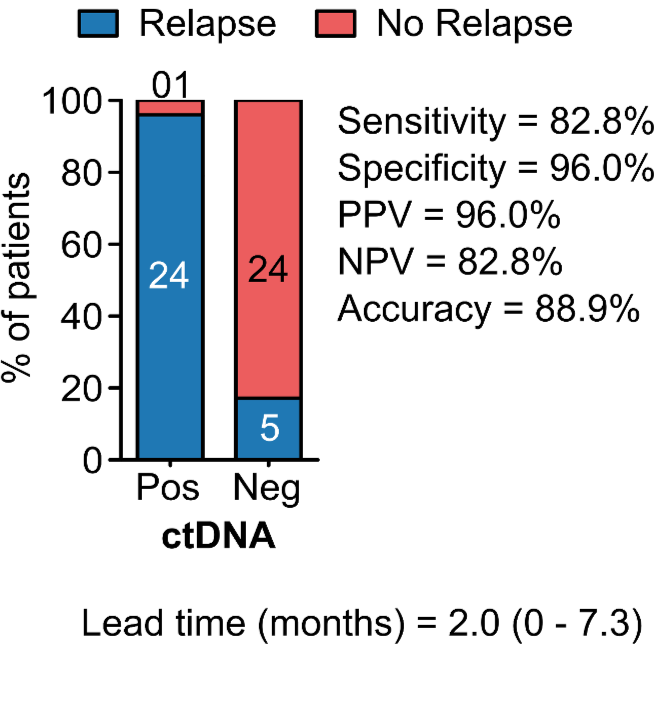
B - Colorectal Cancer (CRC)
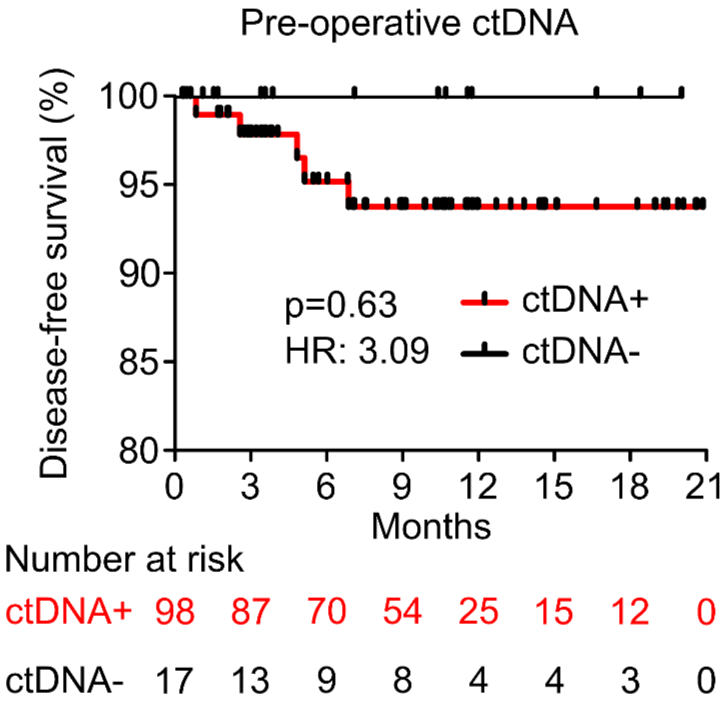
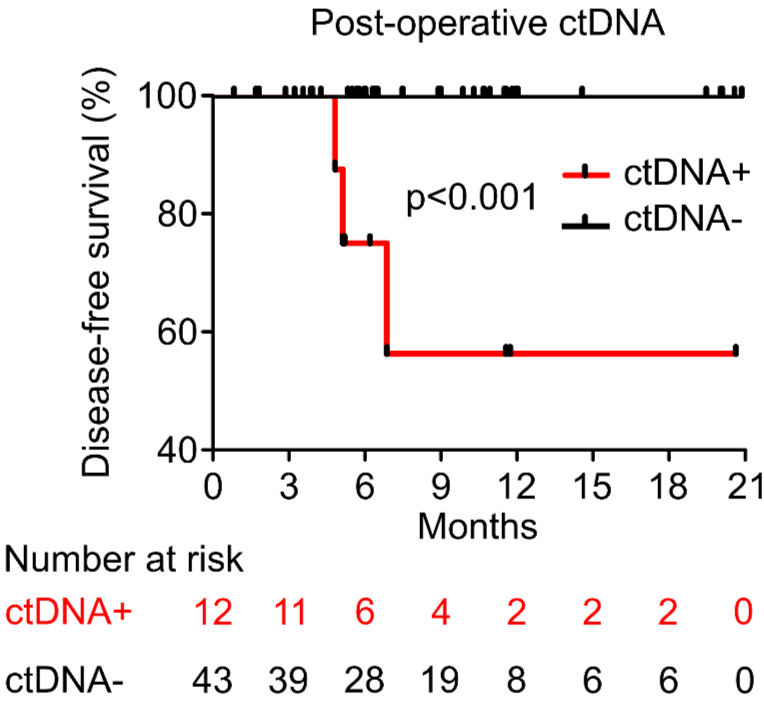
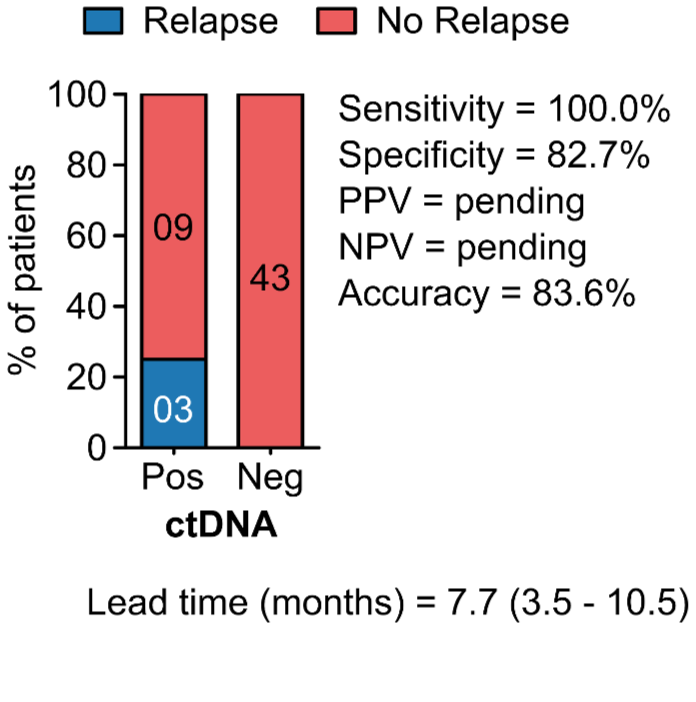
C - Breast Cancer (BC)
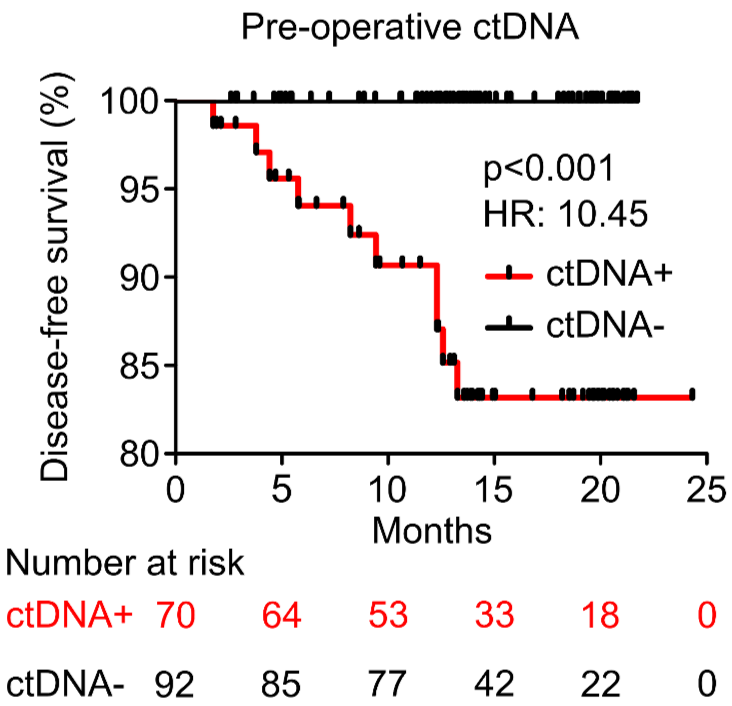
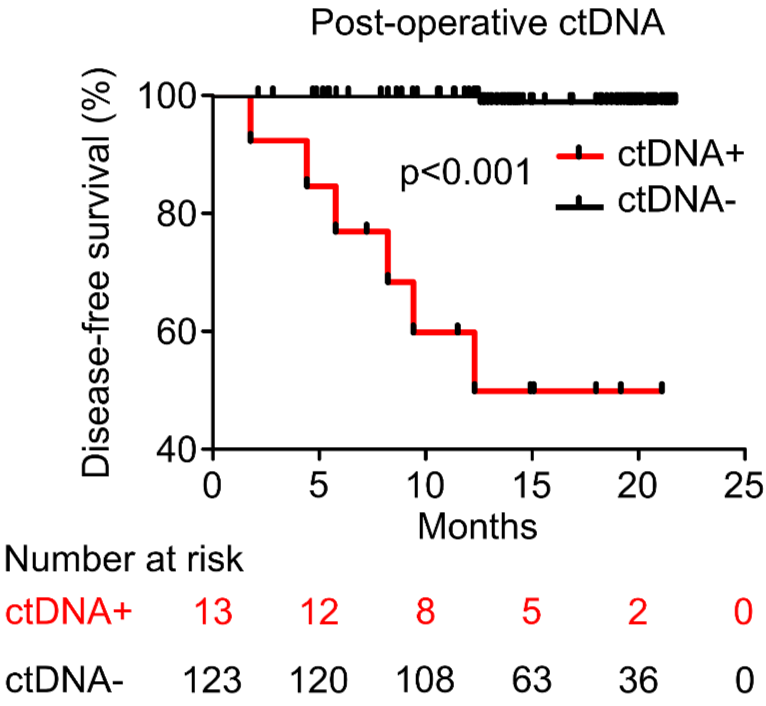
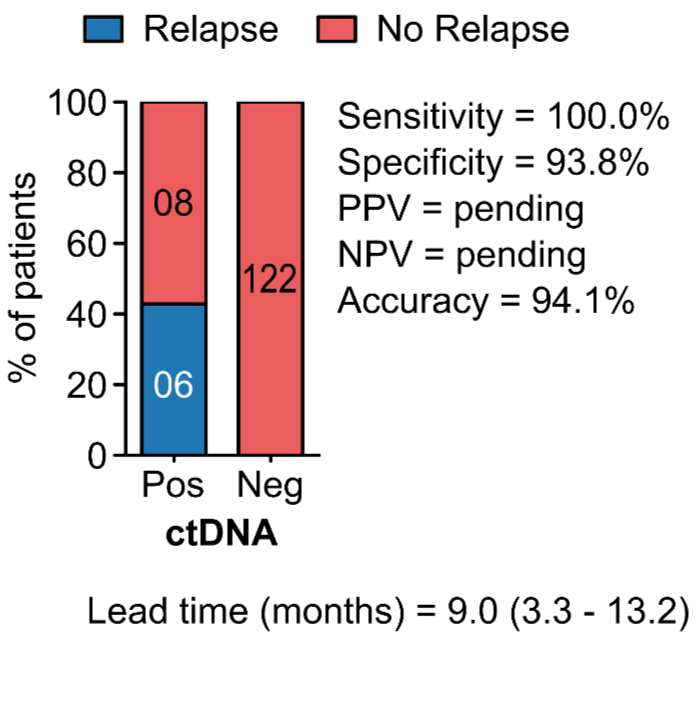
D - Gastric Cancer (GC)
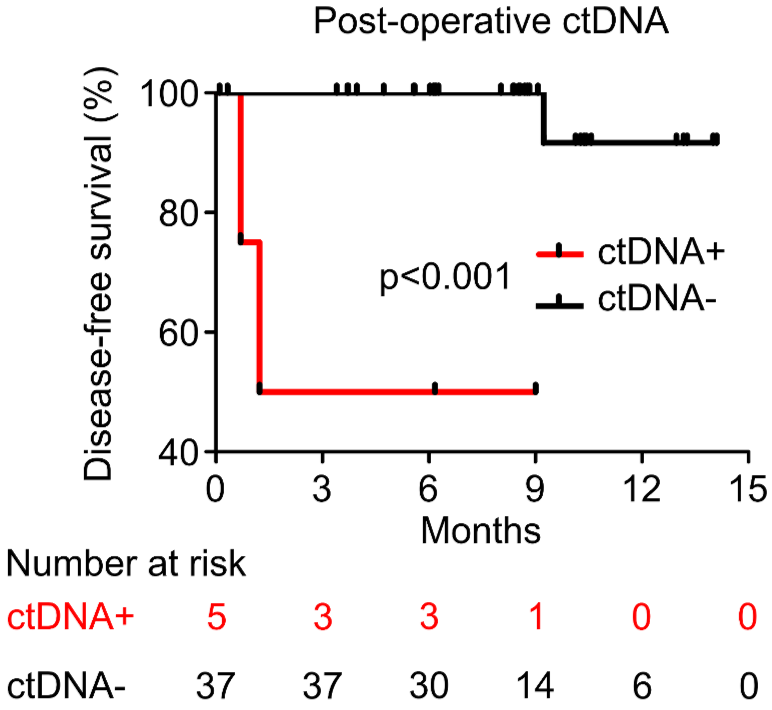
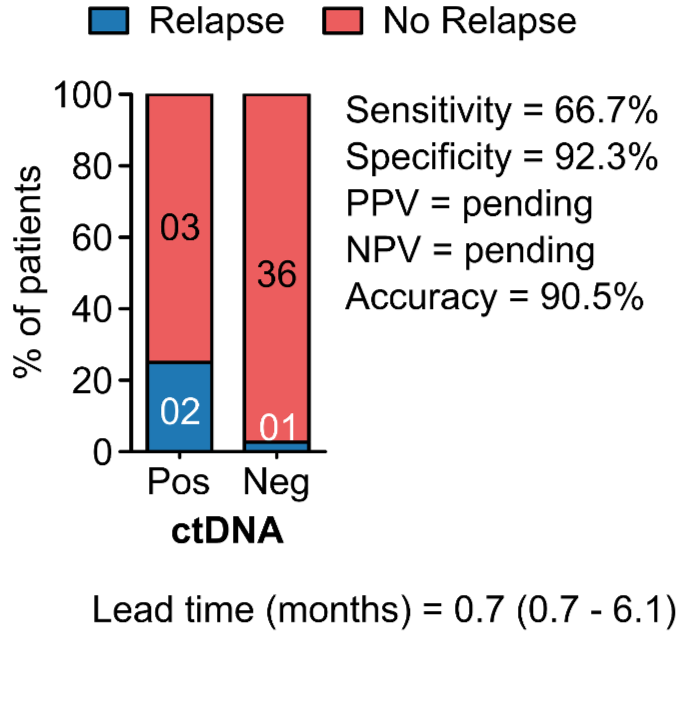
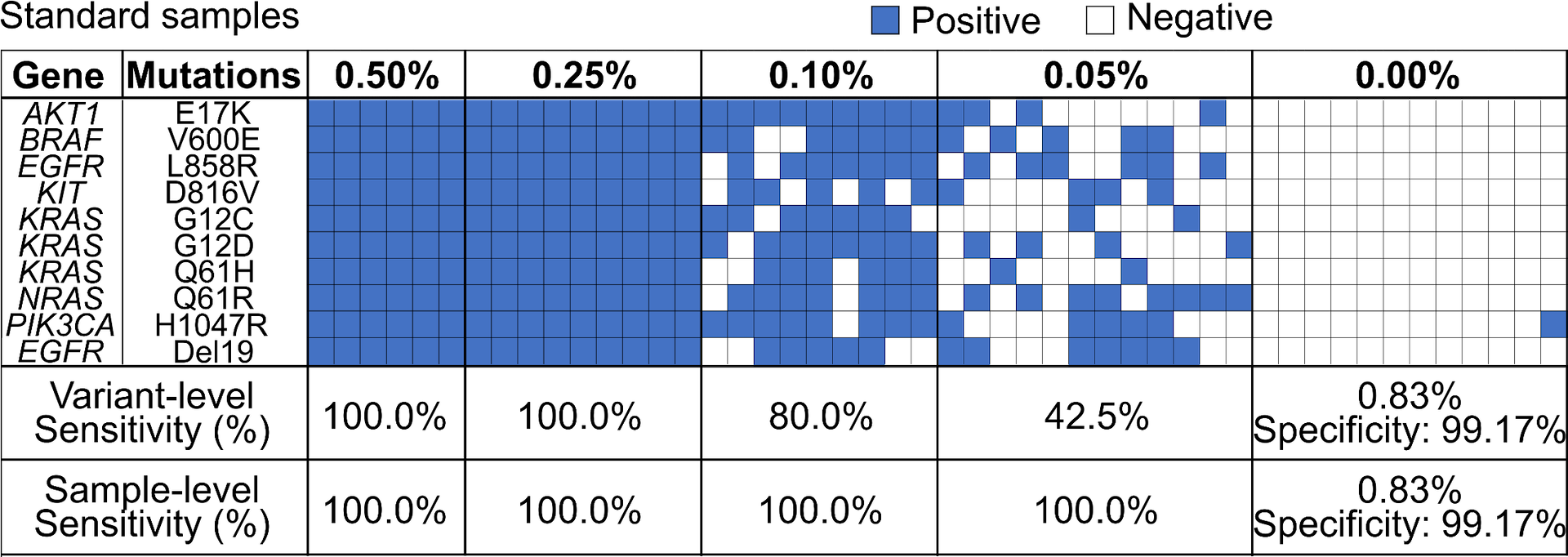
ANALYTICAL VALIDATION
- Limit of detection at VAF ≥ 0.05%
- A sample is defined as ctDNA(+) if one of the mutations has VAF ≥ 0.05%
Ethical approval The study was approved by the institutional ethics committees of the University of Medicine and Pharmacy (#300/HDDD, #81/GCN-HDDD, #14/GCN-HDDD, and 164/HDDD), Thu Duc city Hospital (#17/HDDD), and Cho Ray hospital (# 1183/GCN-HDDD).
Acknowledgement We especially thank the doctors and nurses from UMP, TD, CR hospitals for collaborating with us and all of our patients for participating in the study.
Funding This study was funded by Gene Solutions JSC, Vietnam.
CONCLUSION
- K-TrackTM assay is streamlined and affordable with dual clinical utilities in residual cancer surveillance and actionable mutation profiling.
- The assay could lay foundation to empower precision cancer medicine in Vietnam and developing countries

